

Well, calcium's electron configuration, I could do it in noble gas
#HYDROGEN VALENCE ELECTRONS FULL#
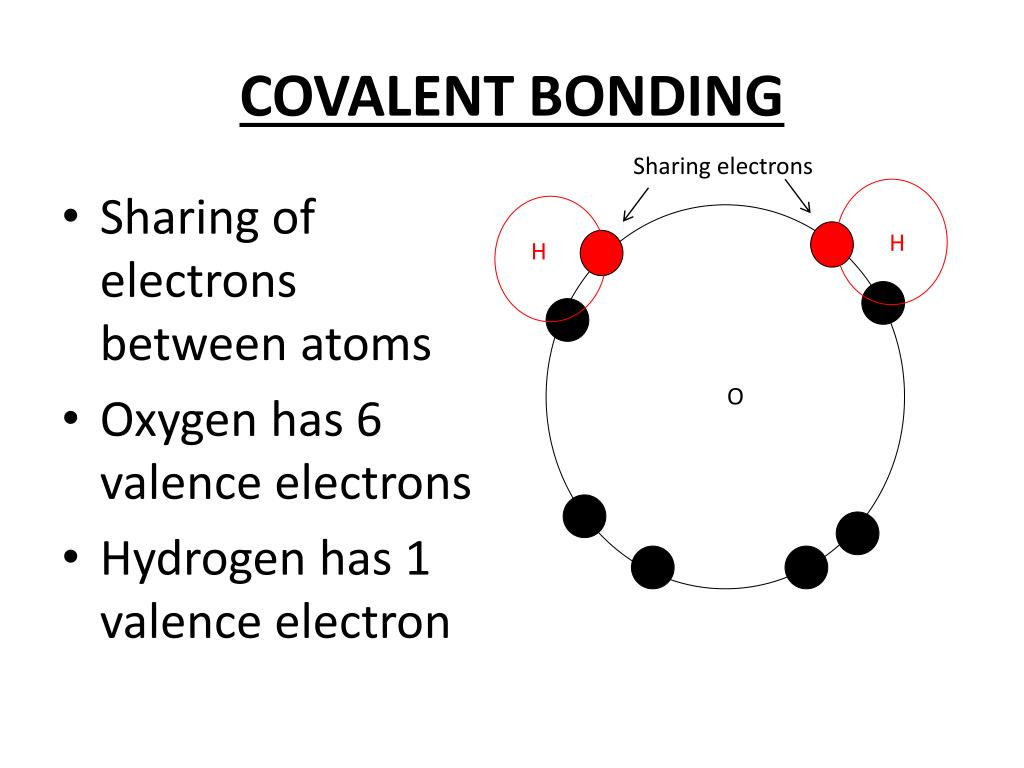
Pause this video, thinkĪbout what the electron configuration of calcium is, and then think about howĬalcium is likely to react given that atoms tend to be more stable when they have a full outer shell, where both their S and P Oxygen does a lot of, it grabs electrons from other things. And so you'd say, alright, well maybe they can grab thoseĮlectrons from something else and that's actually what The 2s and the 2p would beįilled then, we would have 2p6. To share, or get ahold of, two more electrons, because then that outermost shell will have a full number With a Lewis structure, and it might look something like this, where oxygen has one, two, three, four, five, six valence electrons, and you might say, hey, it would be nice if oxygen somehow were able And so in this situation, you say, okay, oxygen has six valence electrons, and oftentimes that's drawn Least a filled SNP subshells in their outer shell. Now, why is six valenceĮlectrons interesting? Well, atoms tend to be more stable when they have a filled outer shell, or in most examples, at Generally aren't reactive, or aren't involved as much in reactions? It has two core, two core electrons. And how many core electrons does it have? And the core electrons In the outermost shell? You have six electrons here. So the outermost shell is beingĭescribed right over here, this second shell. How might oxygen react, it's interesting to look at Up all the electrons here, I have exactly eight electrons. So I have four right now, I have to have four more, so then you're going to have 2p4. Well, in a neutral oxygen atom, you have eight protonsĪnd eight electrons, so first you're gonna fill the one shell, then you are going to startįilling the second shell, so you're gonna go 2s2, So oxygen's electronĬonfiguration is what? Pause this video and see if And so just to make that point, or make it a little bit clearer, let's look at the electron configuration of an element that we'll Or how a given element is likely to react with other atoms. Is, what is the point? And the point of electronĬonfigurations is, is they can give us insights as to how a given atom Might have been asking yourself this whole time that we've been looking at electron configurations So its number of valence electrons is 1.- We are now going to talk about valence electrons, and non-valence electrons, whichĪre known as core electrons and so one question that you Hence it requires combining with one more electron of an atom to achieve stability.

How many Valence electrons does Hydrogen have?Ī Hydrogen atom has only one electron in its outermost shell. And if the electronic configuration of any element is less than or equal to four, its valency can be determined by the group it belongs to in the periodic table. Hence it belongs to the first group of the periodic table. Since Hydrogen belongs to the first group of elements its electronic configuration is 1. For example, four Hydrogen atoms can form a bond with a carbon atom, to form the compound methane (CH 4). Hence one hydrogen atom can form bonds with electrons of many atoms simultaneously. So to achieve stability it needs to share a bond with only one electron of another atom. It can also find out by looking at the periodic table since Hydrogen belongs to group 1.
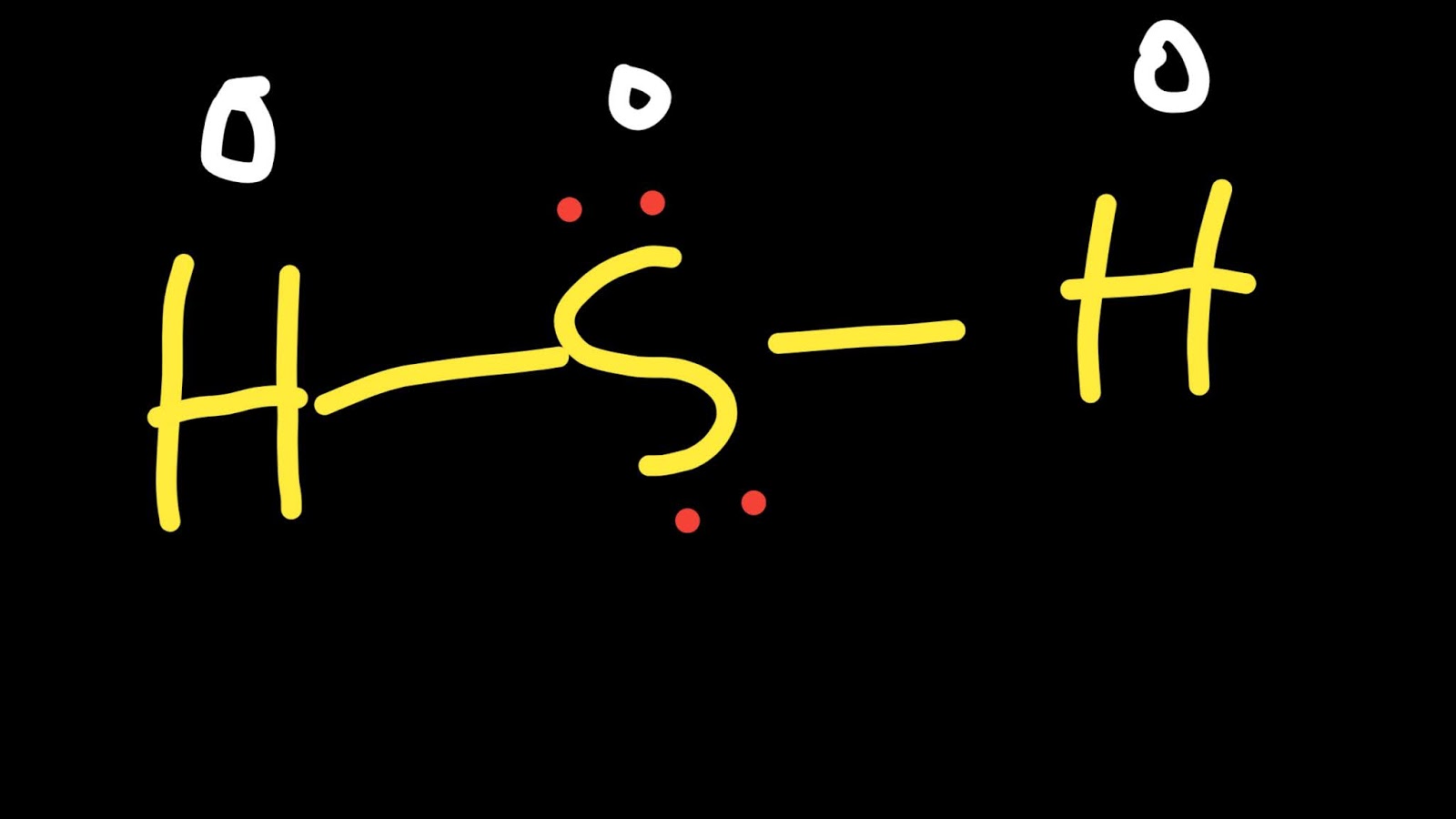
Since Hydrogen has only one electron in its shell, its valency is 1.


 0 kommentar(er)
0 kommentar(er)
